Introduction
A stressor is change in environmental conditions that places stress on the health and functioning of an organism, population and/or ecosystem. Stressors can be either natural or anthropogenic in origin, and either direct (e.g. oxygen deficiencies) or indirect (e.g. lack of food availability due to stresses on prey species) in their effects on an ecosystem.
The vulnerability of different species to stressor effects can depend on factors such as life stage, habitat preference, reproductive cycle and community structure. Stressor effects are detected in ecosystems through measurable response variables, such as changes in ecological status or ecosystem service provision.
Multiple stressor combinations can have a variety of outcomes: additive (i.e. total effects are cumulative); synergistic (i.e. total effects are multiplied) or antagonistic (i.e. total effects are reduced). Stressors can belong to either driver, pressure or state categories in the driver-pressure-state-impact-response (DPSIR) causal framework used for describing the interactions between society and the environment.
To assess the impact of multiple stressor combinations on biodiversity, ecosystem functioning and services is complex. This factsheet introduces some of the key concepts (i.e. interactions, impacts, spatial distribution, management) of multiple stressors without pretending to be comprehensive. The aim is to illustrate the complexity of disentangling the impact of multiple stressors and identifying appropriate management strategies.
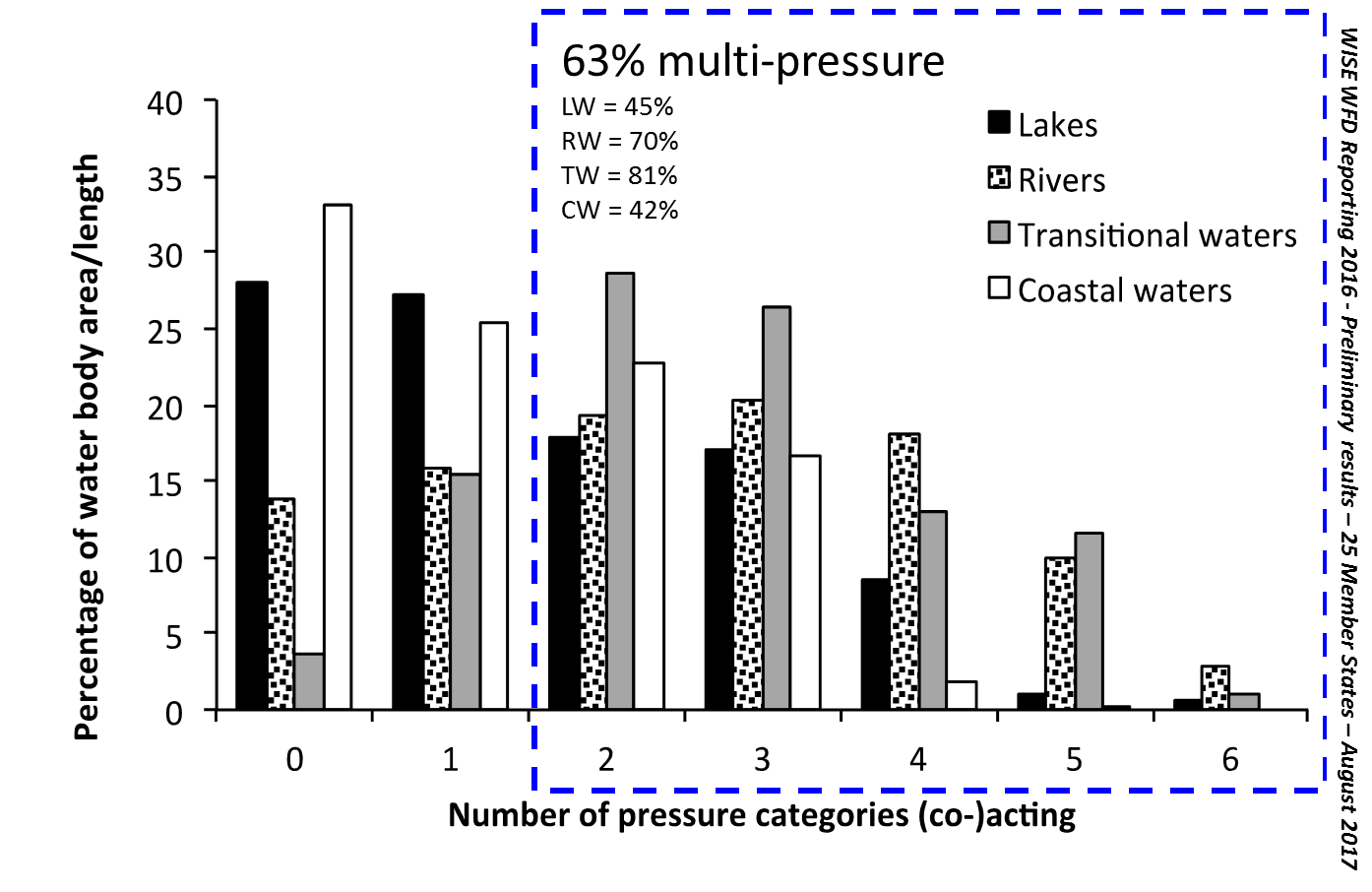
Fig. 2: Percentage of water bodies affected by no, one or multiple pressure categories (WISE WFD Reporting 2016 - Preliminary results – 25 Member States – August 2017).
two-pressure-combination-most-frequently.png)
Fig. 3: Most frequent two-pressure combinations (EEAb, 2012)
Multiple stressors in European water bodies
Based on the official reporting of EU member states for the Water Framework Directive, the most common two-pressure combination acting on lakes and rivers is diffuse water pollution combined with hydromorphological pressures (Figure 1; Figure 2). For example, this might describe a river both fragmented by weirs and dams and subject to nutrient pollution from agricultural fertilisers. In transitional and coastal environments, the most common stressor combination is diffuse pollution with a group of ‘other’ stressors including overfishing, the impact of alien species and waste disposal (EEA 2012a).
Nõges et al. (2016) reviewed the quantitative multi-stress research across surface water and groundwater environments, highlighting commonalities and differences between stressor combinations and response patterns in these systems. The results show that the most prominent two-stressor combination addressed in scientific research resembles the outcomes of the member states’ reporting: pollution (mostly by excess nutrients, but also toxins) paired with hydromorphological modification is the best documented stressor-combination in the scientific literature.
Combined impact of multiple stressors: synergistic, additive or antagonistic?
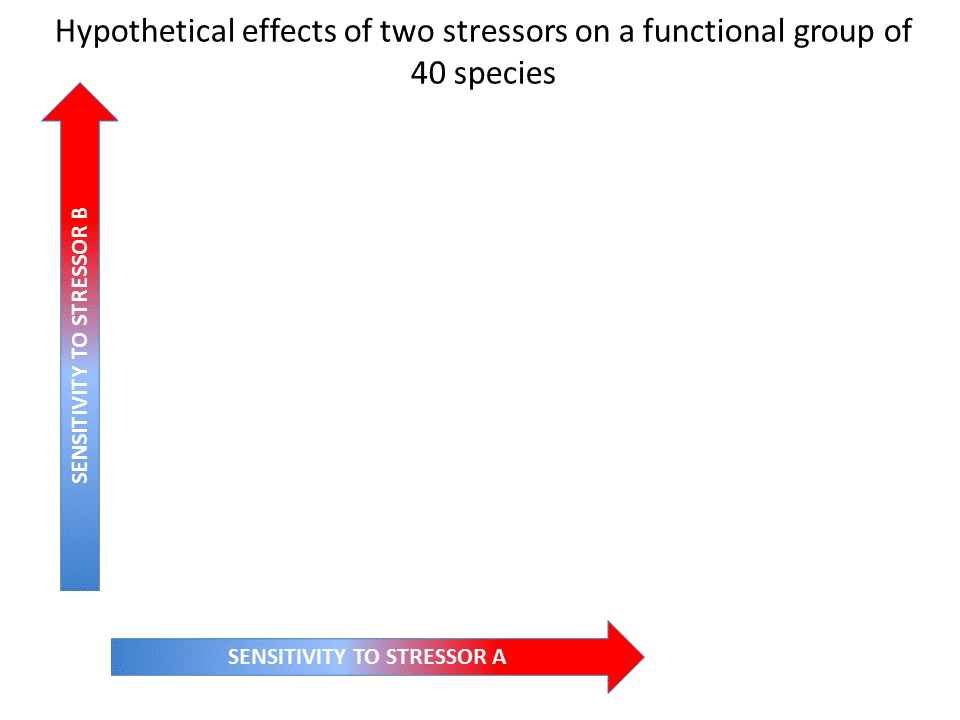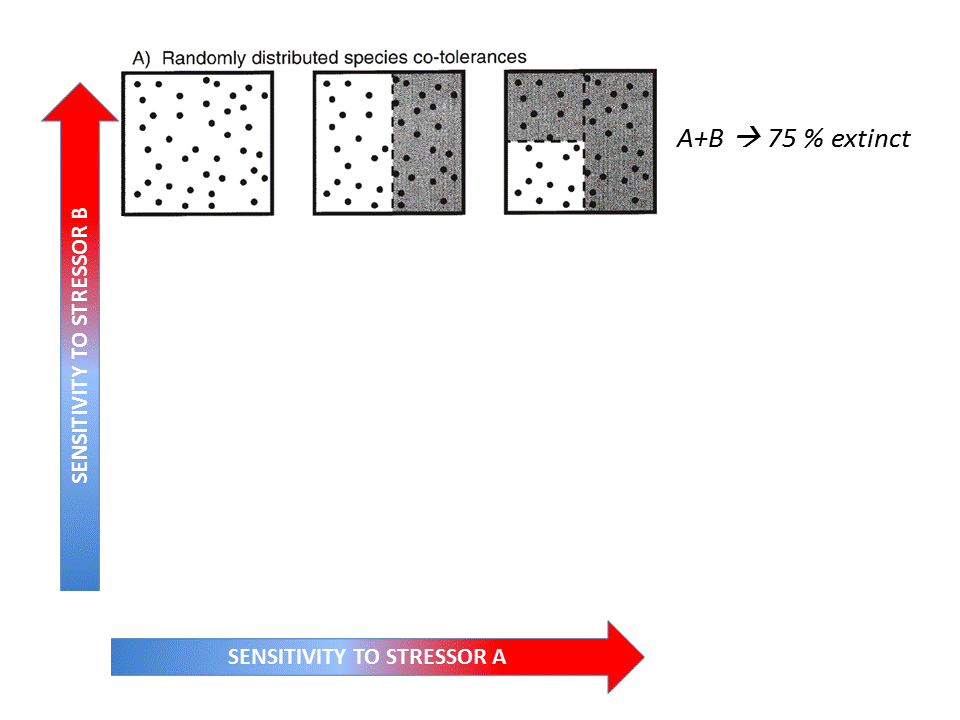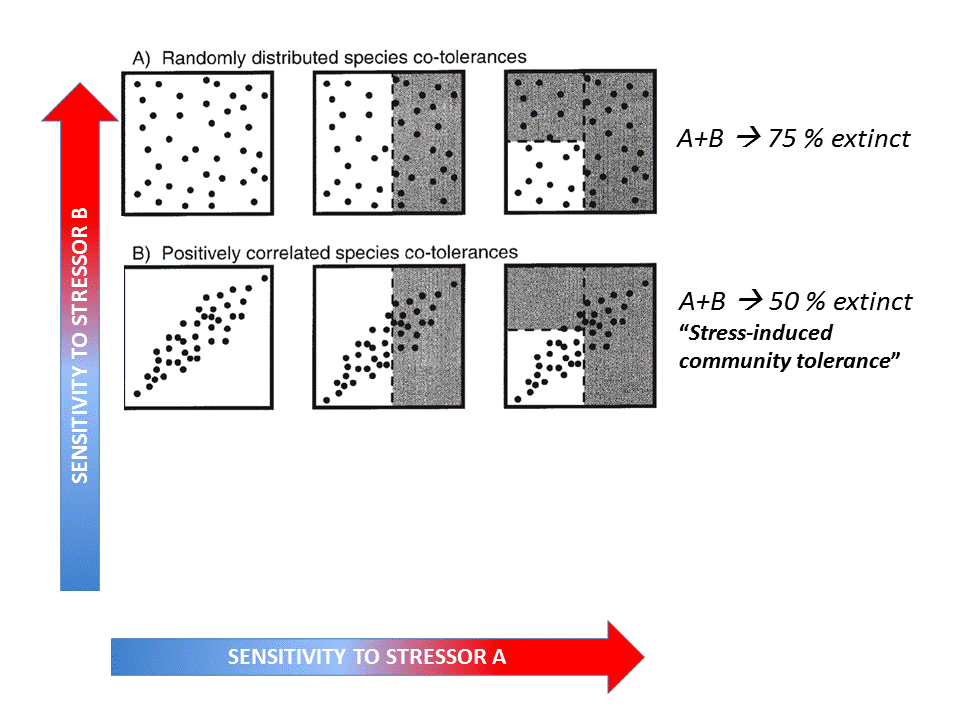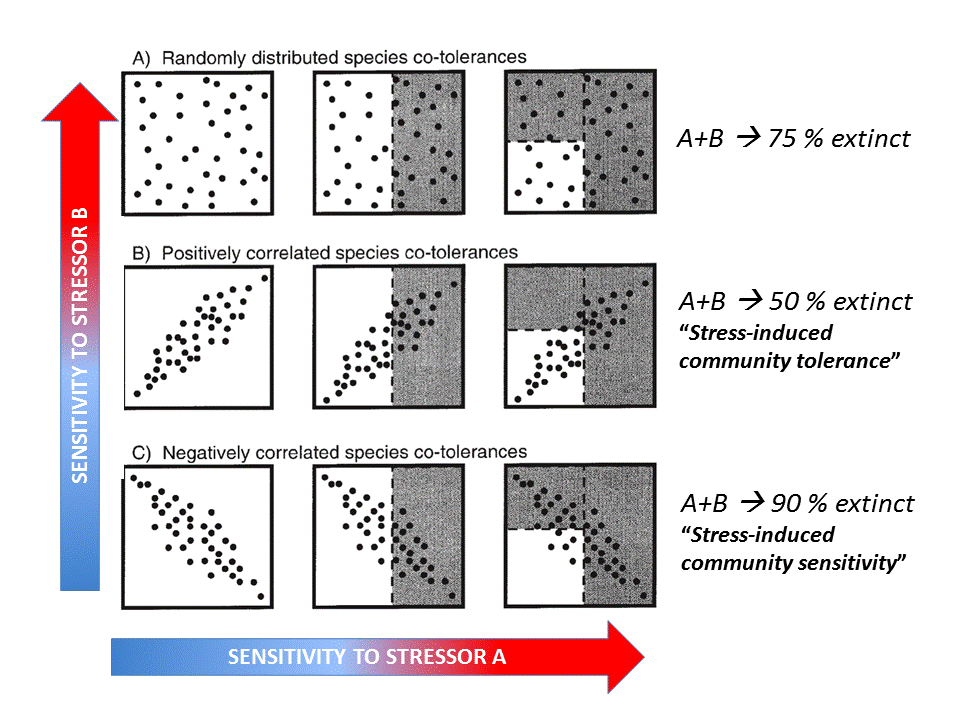
Understanding multiple stressors is particularly challenging when their combined effect cannot be predicted based on evidence from single-stressor studies, i.e. there are interactions that cause non-additive effects (Vinebrooke et al. 2004). Given the increasing multiplicity of environmental stressors associated with global change, there is an urgent need to develop a better understanding of the interactive effects of multiple stressors on ecosystems to better predict their responses to a changing environment. The sign and strength of the correlation between species sensitivities to multiple stressors must be considered when predicting their impacts.

Fig. 4: Hypothetical effects of two stressors on a functional group (Vinebrooke et al. 2004). Click on the image to open the animation.
The conceptual model by Vinebrooke et al. (2004) regarding species sensitivity and tolerance is a useful theoretical introduction whether the impact of multiple stressors may be either synergistic, additive or antagonistic. It shows that the sign and strength of correlations between stressor-tolerances affect the response of biodiversity and ecosystem functioning to multiple stressors.
A recent quantitative meta-analysis of 88 scientific publications investigating 286 paired stressor effects in freshwater ecosystems (Jackson et al. 2016) showed that 56% of the combined effects were antagonistic, while only 28% were synergistic and 19% additive (see the 24/09/2015 Freshwater blog “Ecological surprises: why multiple stressors in freshwaters may cancel each other out”).
Multi-stress effects depend greatly on which response variable is investigated, and the ecosystem type and stressors involved. A possible explanation for more antagonistic responses by freshwater biota to stressors is that the inherent greater environmental variability of smaller aquatic ecosystems fosters greater potential for acclimation and co-adaptation to multiple stressors. These antagonistic interactions were found rather for functional than structural metrics (the latter, e.g. diversity, were mostly additive) supporting the hypothesis of 'functional compensation‘ of stressor effects (see hereafter).
These findings have implications for management of freshwater ecosystems. For stressor pairs that generate additive or synergistic effects, management focusing on a single stressor should render a positive outcome. However, in communities affected antagonistically by stressor-pairs, both stressors may need to be moderated to produce any substantial ecological recovery.
Functional groups and ecological guilds
The insurance hypothesis (Naeem 1998, Yachi & Loreau 1999) is based on the concept of species redundancy and predicts that biological communities comprised of functional groups with many species allow for ecosystem functioning being more resistant to species loss due to anthropogenic stress. This increased resistance results from other species belonging to the same functional group that are tolerant to the stressors causing species loss and thus functionally compensate for the species that have gone extinct. This explains the evidence from literature that the impact of a stressor is less pronounced at the functional level than at the species level (e.g. Jackson et al 2016).
Ecological guilds are considered a useful grouping of individual species based on their similarity in environmental dependencies. Stressors generally affect species belonging to the same guild in a comparable magnitude. By knowing species sensitivities, the stress caused by a pressure can be quantified and summarised in species sensitivity distributions for the entire biological community.
spatial-aspects-of-several-pressures-river-basin.png)
Fig. 5: Scheme illustrating the spatial aspects of several pressures, their impacts and management responses within a catchment and its water bodies. 1 = Diffuse pressure, 2 = Physical alteration of bed, channel and/or floodplain, 3 = Hydrological alteration, 4 = Barrier/dam
Spatial and temporal aspects of multi-stressed river basins
The sources of pressures are often distributed over catchments (Figure 4). Some are widespread (referred to as ‘diffuse’), while others occur at a particular location (‘point’). Their impact will be local, but can also be distant either upstream or downstream. For instance, barriers may have far reaching upstream effects, while water abstraction or pollution may impact water bodies downstream. Thus water bodies within a river basin are affected by combinations of local pressures as well as remote pressures. This is illustrated by the numbers 1 to 4 for single water bodies in Figure 4. Some are impacted by only one single pressure and others by all. It is thus necessary not only to map the origin of the pressures, but also where they have their impact and cause stress (either locally, downstream or upstream).
Such insight is also important when forecasting the potential benefits of restoration measures, which will differ depending whether there are still pressures causing stress in a water body and thereby reducing the effect of measures (illustrated by ‘√’ in Figure 4). For instance, a fish passage (red ‘√’) will allow fish migration to all upstream water bodies. The water body directly upstream the barrier is then still impacted by three pressures (i.e. diffuse pollution, physical alteration, hydrological alteration), while further upstream the impact of only one pressure or none is present. Dam removal (blue ‘√’) will also mitigate the downstream impacts of hydrological alteration.
Besides such spatial aspects, there are also temporal coincidences of pressures (e.g. low flows, high temperatures whereby nutrient and organic load may lead to elevated concentrations). Such concurrence of pressures may cause additional stress. Impact of pressures may also be delayed or long-lasting. For instance, river regulation will alter hydromorphological dynamics causing disequilibrium and a progressive degradation. With disequilibrium is meant that HyMo interventions initiate a change in processes and forces within responses times of years or decades. Example cutting-off meanders and shortening the river course with bank protection or groynes initiates river bed incision which goes on for decades i.e. the morphodynamics of the river are not in equilibrium. Also a dam trapping sediments initiates such changes due to sediment ‘hunger’.
multiple-interactions-within-DPSIR-framework.png)
Fig. 6: An illustration of the multiple interactions within the DPSIR framework (Atkins et al. 2011).
Nested%20DPSIR%20framework.png)
Fig. 7: A Nested DPSIR framework (Atkins et al. 2011).
Nested DPSIR: an approach to effectively manage multiple used and multi-stressed environments
Ecosystems exploited for multiple uses (and consequently impacted by multiple stressors) require a strategy to optimise their management. In this regard, Atkins et al. (2011) proposed a combination of the DPSIR framework with the concept of ecosystem services and societal benefits to create a specific framework to support decision making. The current limits of aquatic ecosystem management requires the coupling of the emerging concepts of DPSIR and the Ecosystem Approach, including ecosystem services and the societal benefits emanating from those services.
The ecosystem approach requires an understanding of the way in which the ecological system functions, while at the same time understanding the way society manages the exploitation of ecosystems. This includes knowledge about the potentially adverse and/or advantageous effects of its activities, comprising mitigation and/or compensation. Ecosystem services have a fundamental link to the DPSIR framework. These are associated with the production and delivery of the benefits for society. The DPSIR approach shows how the process of delivering those benefits can create, for example, adverse State changes and Impacts which require a Response (Figure 5).
Given the often diverse uses and users of aquatic ecosystems, multiple DPSIR cycles are required. The management of such systems is complex, which can be seen in the visualisation of a nested DPSIR framework given in Figure 6. It fully considers the multiple activities undertaken through the DPSIR framework of multiple users within the boundary of the system.
MARS research on multiple stressors
The MARS project has investigated multiple stressors interactions and impacts across various spatial scales: from the European continent (to model future storylines of climate and society); individual river basins (to provide valuable knowledge for water managers); down to small-scale experiments (to unravel stressor impacts on specific biota). The results are summarised in deliverables and scientific publications. Here is a selection; the full overview is available on the MARS website.
- A framework has been developed to select suitable indicators for multi-stressor effects. This resulted in the selection of 15 benchmark indicators (D2.1-3).
- Experiments are useful in generating causal insight into the stress caused by certain pressures on specific (groups of) biota. The results of the MARS experiments (low/high flow, excessive nutrients, fine sediments, low oxygen, hydropeaking, temperature) are summarised in D3.2 and published in >10 scientific publications (Freshwater Blog 2016).
- 16 river basins throughout Europe have been investigated to characterise relationships between multiple stressors and ecological responses, functions and services (Figure 7; D4.1)
- The impacts of multiple stressor combinations on ecological status and ecosystem services have been assessed across Europe (D5.1)
Further reading
MARS publications:
Feld, C.K., P. Segurado & C. Gutiérrez-Cánovas (2016). Analysing the impact of multiple stressors in aquatic biomonitoring data: A 'cookbook' with applications in R, Science of The Total Environment, 573, 1320-1339. DOI: 10.1016/j.scitotenv.2016.06.243 (Read abstract)
Nõges P., Argillier C., Borja Á., Garmendia J.M., Hanganu J., Kodeš V., Pletterbauer F., Sagouis A. & S. Birk (2016). Quantified biotic and abiotic responses to multiple stress in freshwater, marine and ground waters. Science of the Total Environment, 540, 43-52. DOI: 10.1016/j.scitotenv.2015.06.045 (Read abstract)
Reports and publications:
Atkins J. P., Burdon D., Elliott M., & A. J. Gregory (2011). Management of the marine environment: integrating ecosystem services and societal benefits with the DPSIR framework in a systems approach. Marine Pollution Bulletin, 62(2), 215-226. (Read abstract)
EEA (2012a). European waters - assessment of status and pressures - (Download report, 28mb)
EEA (2012b). WISE WFD database of the Member States and the Commission of the European Union, published 12 nov 2012. (External website)
Jackson M.C., Loewen C.J.G., Vinebrooke R. D. & C. T. Chimimba (2016). Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob Change Biol, 22: 180–189. DOI:10.1111/gcb.13028. (Read abstract)
Naeem S. (1998). Species Redundancy and Ecosystem Reliability. Conservation Biology, 12: 39–45. DOI:10.1111/j.1523-1739.1998.96379.x. (Read abstract)
Vinebrooke R.D., Cottingham K.L., Norberg J., Scheffer M.S., Dodson S.I., Maberly S.C. & U. Sommer (2004). Impacts of multiple stressors on biodiversity and ecosystem functioning: The role of species co‐tolerance. Oikos, 104(3), 451-457. (Read abstract)
WISE WFD Reporting 2016 - WISE WFD database of the Member States and the Commission of the European Union, Preliminary results o5 25 Member States, August 2017.
Yachi S. & M. Loreau (1999). Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceedings of the National Academy of Sciences, 96(4), 1463-1468. (Read abstract)
Selected Freshwater Blogs:
Freshwaterblog (2016). Multiple stressors in Science of the Total Environment (External website)
Freshwaterblog (2015). Ecological surprises: why multiple stressors in freshwaters may cancel each other out (External website)
Freshwaterblog (2015b). New journal special issue focuses on river conservation under multiple stresses (External website)
Selected MARS deliverables:
To download to MARS deliverables please visit the project page (External website)
Deliverable 2.1 Four manuscripts on the multiple stressor framework:
- Part 1: Review of multiple stressors and their effects on European surface waters
- Part 3: Framework to select indicators of multi-stressor effects for European river basin management
Deliverable 3.2 Manuscripts on experimental results
Deliverable 4.1 Case study synthesis - Final Report
Deliverable 5.1 Reports on stressor classification and effects at the European scale
- 1-1 Part 1: Multi-stressors on surface water and effects on ecological status
- 1-1 Part 2: Analysis of pressure - response relations: classification of multiple pressures on broad river types
- 1-3: Impact of multi-stressors on ecosystem services and their monetary value
- 1-4: Effects of multiple stressors on ecosystem structure and services of phytoplankton and macrophytes in European lakes





-case-studies.png)